
Sensorion: Promises of Further Gene Therapy Development with Pasteur
January 2024. Sensorion, a clinical-stage biotechnology company specializing in hearing loss disorders, announced that the research partnership framework agreement signed in 2019 with Institut Pasteur at Paris (FR) has been extended up to December 31, 2028, to promote additional development of gene therapy programs. Pasteur grants Sensorion an option for exclusive licenses to develop and market gene therapy drug candidates from collaborative projects.
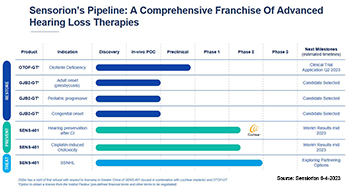
Two gene therapy development programs are currently being conducted under this agreement, including SENS-501 (OTOF-GT) and GJB2-GT. SENS-501, the most advanced program within the partnership that targets deafness caused by mutations in the gene coding for otoferlin, defined as a priority in 2019, has met its objectives. GJB2-GT targets deafness linked to mutations in the GJB2 gene, the most common form of childhood deafness. Three indications, all linked to GJB2 mutations, are currently being evaluated: early presbycusis, progressive hearing loss during childhood, and congenital hearing loss.
Sensorion submitted a Clinical Trial Application (Audiogene) in July 2023 for SENS-501 to initiate a Phase 1/2 clinical trial in the United Kingdom (UK) and in the European Union (EU).
The research partnership successfully led to a second gene therapy program with GJB2-GT, announced in 2021, for which a drug candidate, selected in April 2023, is currently in preclinical development.
Source: Institut Pasteur
Disclaimer: You agree that B2Bioworld is not responsible and will not be held liable for any third party content on its sites or any third-party content, products or services available on other web sites accessed through links from B2Bioworld sites. Links to third-party sites are for your convenience only, and their inclusion on B2Bioworld\'s sites does not imply any endorsement, guarantee, warranty or representation by B2Bioworld.
B2Bioworld Comment
 Sensorion S.A. is a French company headquartered at Montpellier close to Sanofi’s research site. In the past the company tested small molecules (for example Arazasetron besylate, or SENS-401-201). For SENS 401 it performed five clinical trials (EudraCT#: 2018-000812-47) in Bulgaria 8 subjects), Czech Republic (9), Germany (2), Slovakia (12) and the UK (1). In addition subjects were enrolled in Israel (31), Serbia (12), Turkey (10), and Canada (4) totaling 115 patients (out of 301 planned to recruit) between 18 to 64 years (98) and 65 to 84 years (17). Starting in February 2019 trials for SENS-401 were completed in the European Union January 2022. There were no global interruptions, but several amendments to the protocol which decreased the sample size. According to EudraCT no results were communicated.
Sensorion S.A. is a French company headquartered at Montpellier close to Sanofi’s research site. In the past the company tested small molecules (for example Arazasetron besylate, or SENS-401-201). For SENS 401 it performed five clinical trials (EudraCT#: 2018-000812-47) in Bulgaria 8 subjects), Czech Republic (9), Germany (2), Slovakia (12) and the UK (1). In addition subjects were enrolled in Israel (31), Serbia (12), Turkey (10), and Canada (4) totaling 115 patients (out of 301 planned to recruit) between 18 to 64 years (98) and 65 to 84 years (17). Starting in February 2019 trials for SENS-401 were completed in the European Union January 2022. There were no global interruptions, but several amendments to the protocol which decreased the sample size. According to EudraCT no results were communicated.
 Recently, Sensorion has been commercially developing gene therapeutic research of Pasteur Institute’s Institut de l’Audition and surgical techniques 1) developed at Hôpital Necker, both Paris. Apart of presentations at conferences of preclinical results, for example at the American Society of Gene & Cell Therapy in October 2023 2), to date no respective advanced therapy trial, i.e. «SENS-501» is registered in the European Trial Register.
Recently, Sensorion has been commercially developing gene therapeutic research of Pasteur Institute’s Institut de l’Audition and surgical techniques 1) developed at Hôpital Necker, both Paris. Apart of presentations at conferences of preclinical results, for example at the American Society of Gene & Cell Therapy in October 2023 2), to date no respective advanced therapy trial, i.e. «SENS-501» is registered in the European Trial Register.
1) Combining cochlear implant and stapledectomy.
2) Guillaume O. et al.(2023): Preclinical development of an Adeno Associated Vector-Based Gene Therapy (SENS-501) for the Autosomal Recessive Non-Syndromic Deafness 9 (DFNB9). Poster, 26th Annual Meeting of the American Society for Cell & Gene Therapy. Los Angeles, May 16-20.
Other articles recommended
B2Bioworld offers you background information
Human Voices Revalued
On voice-assistants, clinical trials, pathologies, and the COVID-19 bet. Includes interview with Guy Fagherazzi, Luxembourg Institute of Health
Pfizer Embraces Regenerative Medicine
Ruth McKernan, CSO Pfizer Regenerative Medicine on the beginnings and competitors
Sanofi: Triggering Regenerative Functions
Kurt Stoeckli, Global Head of Biological Sciences / Discovery Sanofi Aventis
Is that worth a dollar, or a million dollars?
Ed Torres, Managing Director of Lilly Ventures on the Fund’s approach and input to Eli Lilly’s business development